
Empowering Medical Innovation & Global Healing
At DRMRC, we ensure the generation of accurate and precise findings by sourcing clinical-grade biomaterials, coordinating scientific research, and partnering with the world’s top pharmaceutical leaders.

Coordinating Scientific Researches that Promote Life-Saving Medications

Inspection Services
Third-party verification for materials, facilities, and clinical trials.
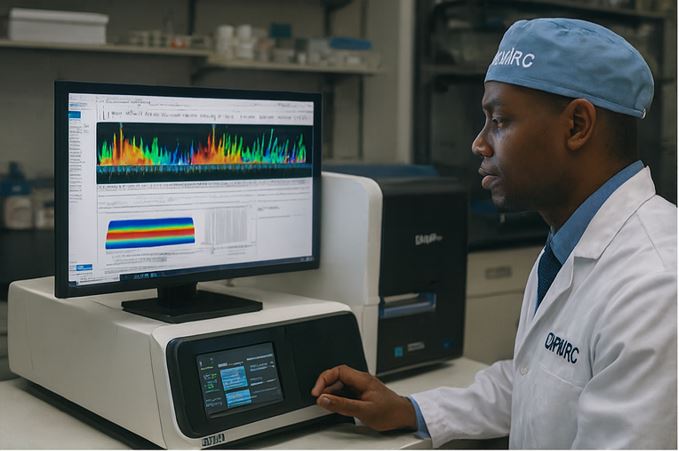
Biomedical Sourcing
Rare & potent clinical-grade materials for drug development pipelines.
Advancing Clinical Evidence with Global Partners
We collaborate with pharma, biotech, NGOs and academic labs to deliver rigorous, ethical research—from protocol to post-trial analysis.

Certified Procurement of Clinical-Grade Biomaterials
Rooted in science, trusted globally. DRMRC is a research-led, procurement-focused organization specialising in the global supply of biomaterials and collaboration on medical trials and drug development.
Companion Intelligence for Faster, Safer Clinical Decisions
Our internal AI workflows help screen sourcing risks, rank evidence quality, flag protocol deviations, and prioritize inspection tasks. The model does not replace expert judgement—it accelerates it with explainable insights.
- Signal triage on supplier dossiers & CoAs
- Automated consistency checks across lab data
- Deviation & SAE trend detection across sites
- Contextual literature scans mapped to protocols
Tailored Engagement for Each Stakeholder
Pharma & Biotech
Secure procurement, inspection, and evidence support to de-risk pipelines.
Partner with DRMRCClinical Trial Sponsors
Protocol design, site monitoring, and post-trial reporting with compliance.
Explore researchMinistries & NGOs
Scalable programs, ethical sourcing, and transparent inspection models.
About DRMRCFrom Discovery to Verified Delivery
Trusted Globally. Rooted in Science.
Founded in 2012 in London, UK. Research-led, procurement-focused. We support pharmaceutical manufacturers, clinical trial sponsors, biomedical innovators, health ministries, NGOs, and academic labs.
- Audit-ready documentation and chain-of-custody
- Independent verification for labs, sites, and trials
- Ethical sourcing commitments and transparency

Where Our Network Delivers Outcomes
Rapid Dossier Triage
AI signal-triage reduced manual screening time by 48% while increasing flagged inconsistencies.
Read moreInspection Prioritization
Risk-weighted inspection routing improved first-pass compliance in new Supplier cohorts.
See servicesEvidence Consistency
Cross-study variance checks surfaced protocol deviations early for corrective action.
About DRMRCOur Global Footprint (Live counters)
Explainable AI for Faster Decisions
Companion intelligence to triage supplier dossiers, surface protocol deviations, and prioritize inspections—augmenting expert judgement.
- Deviation & SAE trend detection
- Evidence quality scoring
- Automated consistency checks
Privacy-Preserving, Federated Insights
Site data stays local while models travel—enabling multi-center learning without sharing raw patient data.
- Federated learning across labs
- Synthetic control arms
- Bias & drift monitoring
From Bench to Bedside at Scale
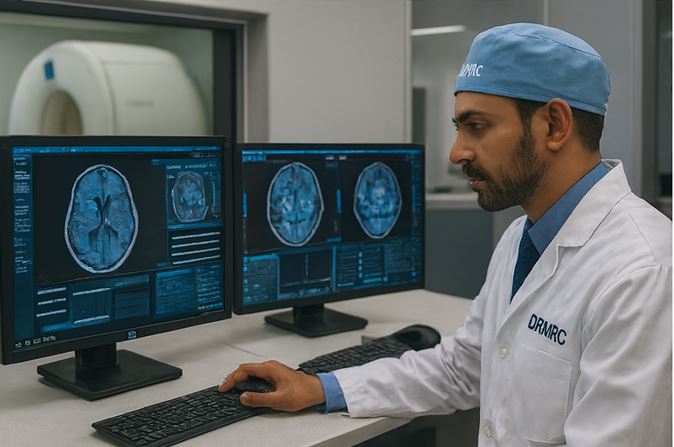
Combine EHR, imaging, genomics, and lab signals to generate robust real-world evidence and accelerate translational medicine.
- Multi-omics data harmonization
- Imaging AI (CT/MRI pathology cues)
- Auto-QC pipelines & lab robotics
CRISPR QC • Generative Protein Design • Spatial Omics • Digital Pathology • mRNA Platforms
We partner across cutting-edge domains—combining validated lab workflows with AI-driven analysis to translate discoveries into clinical impact.
Our Clients & Collaborators
From Big Pharma to biotech startups, DRMRC supports
- Pharmaceutical manufacturers
- Clinical trial sponsors
- Biomedical innovators
- Health ministries & NGOs
- Academic and institutional labs
